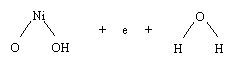Batteries for Mobile Robots
1. Chemical Energy
Mobile robots move – therefore we need to
consider the important question of where the energy required for locomotion
is going to come from. In the case of large robots we would like to be able
to drain energy at high peak rates. For smaller robots, we would like to have
enough energy for several hours of continuous work. Internal combustion engines
are out of the question, since the robots we are considering will be used
indoors. Fuel cells, which combine hydrogen and oxygen, and do not produce
toxic gases, will be an interesting source of energy in the future, but the
technology has not yet matured for the kind of applications we consider here.
Therefore we need to use some form of electrical energy stored in rechargeable
batteries.
The electrical battery was invented several
centuries ago. Rechargeable batteries have been around for many decades and
are improved continually, from year to year. A battery consists of a negative
and a positive electrode. Each electrode is made of a different material and
there is a chemical reaction in the interior of the battery, a different one
for each electrode but complementary. A modern battery can be understood,
as a first approximation, as a kind of sandwich in which the top and bottom
materials are the electrodes, and the material in the middle is the electrolyte.
The electrolyte makes possible the chemical reactions inside the battery and
transports ions from one electrode to the other. The electrolyte is also a
reservoir of ions for the chemical reactions needed at the electrodes.
Summarizing: a battery transforms the chemical
energy stored in the battery’s material in electricity. Batteries which cannot
be recharged are called primary batteries; rechargeable ones are called secondary
batteries. We are interested here in rechargeable batteries, since primary
batteries are too expensive in the long run.
2. Electrolytes
There are many spontaneous chemical phenomena
triggered by the contact of one substance with another. Many salts, for example,
readily dissolve in water and form ions. Table salt consists of sodium (Na)
and chloride (Cl). When put into water, the sodium atoms separate from the
chloride atoms. Water molecules are tightly bound whereas NaCl has weak ionic
bonds – one electron is more tightly bound to the chloride than to the sodium.
Also, water is a polar liquid, that means, water molecules have a positive and a negative
side because the electrons charge is not distributed uniformly in the molecule.
Water molecules “stick” to each other because of their polarity.
When NaCl dissolves in water, water molecules
surround the sodium ion (Na+) neutralizing its charge with their negative
tails. The chloride ion (Cl-) is neutralized by water atoms surrounding it
with their positive sides oriented towards the chloride. NaCL is called an
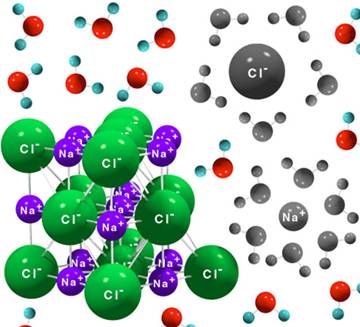
electrolyte because it dissociates in ions in water. Figure 1 shows an NaCl ionic crystal dissolving into water. Water molecules
are negative on the oxygen side, positive to the side of the two hydrogen
atoms.
We are interested in electrolytes because
they can make water conductive and also because many kinds of batteries depend
on ionic reactions. The necessary ions are kept in water, wrapped in water
molecules and ready to be used for a certain chemical reactions.
3. Redox reactions
A battery is capable of delivering current,
i.e. a continuous electron flow, because the material used to build the negative
electrode releases electrons during a chemical reaction
whereas the material
in the positive electrode absorbs them. The negative electrode is called the
anode, the positive one is the cathode. This means that a chemical reaction
is releasing electrons at the anode and another chemical reaction is consuming
them at the cathode. Different types of batteries differ in the kind of reactions
in their interiors and industry is always looking for new technologies that
can deliver more stored energy for each gram of battery.
In a battery, the anode and cathode are
separated by the electrolyte, whose only mission is to transport ions from
one side to the other. The electrolyte can be purely liquid (as in car batteries)
or can be put inside a porous material which also acts as electrode separator.
The material in the negative electrode has to lose electrons - this is called
oxidation. The material in the positive electrode has to absorb electrons
- this is called reduction. For a battery we need two chemical reactions,
an oxidation and reduction pair, also called a redox reaction pair. The specific
example of NiCd batteries can make this clear.
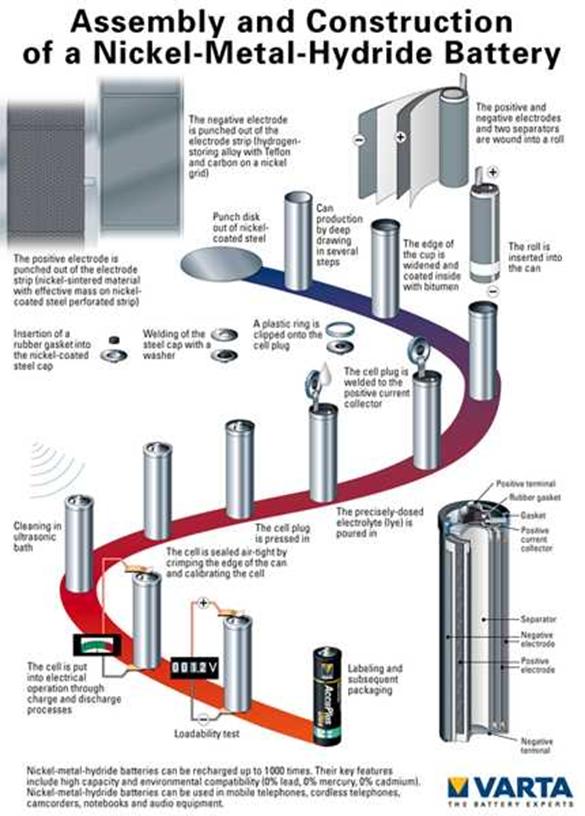
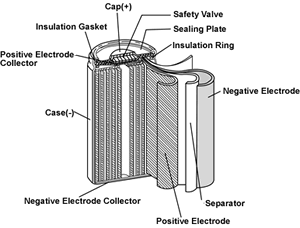
The diagram shows a picture of a cylindrical
NiCd battery. The negative and positive electrode are
sheets of material set apart by a separator soaked with electrolyte. The three
sheets are rolled as a cylinder, one of them is connected
to the positive, one to the negative collector. There is safety valve to eliminate
excess pressure from the chemical reactions.
In a charged NiCd battery, the cathode contains
nickel oxyhydroxide (NiOOH) and the anode contains cadmium. The electrolyte
consists of KOH, that is liquid potassium hydroxide.
Cadmium atoms at the anode dissolve spontaneously in the electrolyte and transform
in positive ions, releasing two electrons at the same time. This chemical
reaction is expressed as:
Cd
-> Cd+2 + 2e-
The cadmium ions combine immediately with
the OH- ions present in the electrolyte to form Cd(OH)2.:
Cd+2
+ OH- + OH- ->
Cd(OH)2
The chemical reaction at the anode can continue
because the electrons move out of the anode the moment an electric device
is connected to the negative and to the positive electrode. A current is established
and electrons flow towards the cathode.
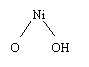
At the cathode there is another spontaneous
reaction during battery discharge. Two electrons combine with nickel oxyhydroxide
and water to form nickel hydroxide:
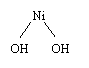
NiOOH + NiOOH
+ 2e + H2O + H2O -> Ni(OH)2 + Ni(OH)2
+ OH- + OH-
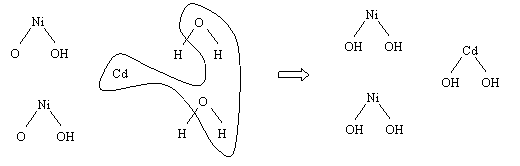
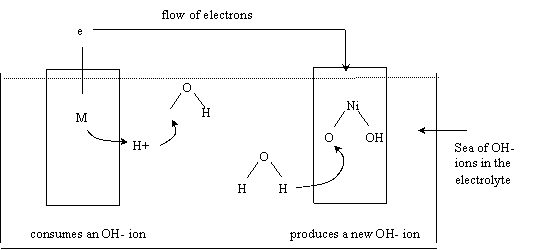
The two reactions can be better visualized with the
following picture:
The curved line shows the reaction taking
place in the anode. There is a flow of two electrons from the anode to the
cathode.
During recharge of the battery the direction
of the arrow is inverted: the reactions are reversible and the original structure
of the electrodes is restored. The battery charger forces a current in the
inverse direction as during discharge and reverses the chemical reactions.
Of course this can be done only a fexied number of times, before the battery has deteriorated so badly that it
becomes unusable.
The main alternative to
NiCd batteries for small mobile robots are NiMH batteries. The positive
electrode contains nickel again, while the negative electrode contains some
kind of metallic alloy that can absorb hydrogen. Some alloys are very good
at that, absorbing many hydrogen atoms in its structure.
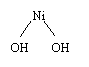
In NiMH batteries the reaction at the positive
electrode is exactly the same as in the case of NiCd batteries, as shown in
the figure. A water molecule is ionized, a proton and the electron attach
to NiOOH to form Ni(OH)2.
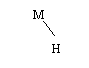
The reaction in the negative electrode takes
place when the metal releases the stored hydrogen and combines it with an
OH- ion to form water, setting free an electron:
MH
+ OH- -> M +
H2O + e-
The complete redox pair is similar to NiCd
batteries:
The formula of the redox pair obscures the
fact that electron transport takes place between the anode and cathode and
that the ion OH- is being also produced and absorbed at the electrodes. Since the transport
of electrons is very fast and since the transport of ions very slow, a reserve
of OH- ions is needed in the electrolyte. This is provided by the dissolved
potassium hydroxide, KOH.
4. Issues of battery
selection
The batteries used for mobile robots must
have a set of desired characteristics that limit the choices available. The
main issues are:
-
Geometry of the batteries. The shape of the batteries can be an important characteristic
according to the form of the robots.
-
Durability. We would
like to be able to recharge the batteries many times in order save costs.
-
Capacity. The capacity
of the battery pack in milliamperes-hour is important. It determines how long
the robot will run until a new charge is needed.
-
Initial cost. This is an
important parameter, but a higher initial cost can be offset by a longer expected
life.
-
Environmental factors. Used batteries
have to be disposed of and some of them contain toxic materials.
As an example: the batteries used in the
FU-Fighters 2002 robotic soccer team were packs of 8 NiMH units with a nominal
capacity of 2000 mAh. With this batteries the robots
could be used for several hours. The
size of the batteries allows them to be placed at the bootom of the robot.

5. Comparison of battery
types
Rechargeable batteries differ in several
important respects according to the technology used. In robotics, we are interested
in a batteries with high energy density, that is,
they should provide much power and be of low weight. NiCD and NiMH are the
most popular rechargeable batteries for robots, while Li-ion batteries are
used in laptops. The new Li-ion polymer and reusable alkaline batteries have
characteristics that could make them interesting in the future.
Charge
We will compare in what follows the NiCd
against the NiMH batteries. The first important parameter is how do both batteries charge. Figure x and y
show the charging curves for NiMH and NiCd batteries, respectively.
There are three curves in figure x. If the
nominal capacity of a battery is 160 mAh, for example, then 160 mA is called
a “C”. A C is the current that can be delivered by this specific battery during
an hour. Batteries can be charged by circulating through the battery a current
of one C, more than one C or a fraction of a C. Usually a fraction of a C
is used and this means that several hours are needed until the battery is
fully charged. The curve for NiMH batteries (Panasonic HHR 160A) shows that
the voltage increases slightly when the cell is gradually charged. These batteries
can be overcharged with 20% more energy than its nominal capacity. A charge
with one C leads to a slightly higher voltage in the cell as a charge with
1/3 C.
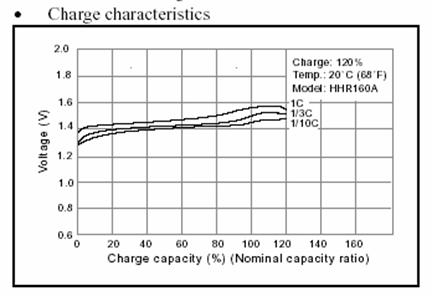
NiMH charging curve with three
different currents
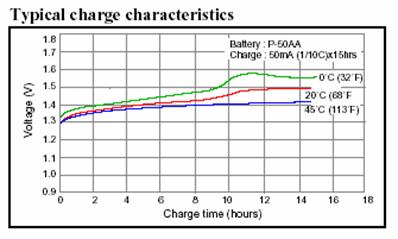
The next curve, for NiCd batteries, shows
that the voltage reached by the batteries is also affected by the temperature.
At 45 degrees Celsius the final voltage is 1.4 volts for each cell, at 0 degrees
it is more than 1.5 V.
Discharge
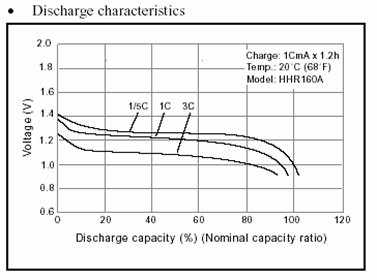
Both NiCd and NiMH batteries have a flat
discharge curve. That means that the energy is extracted from the batteries
and the voltage only drops significantly when the capacity limit has been
reached. This is important for applications, since we would not like voltage
to degrade continuously as the battery’s energy is consumed. The curve shows
that if energy is extracted at a rate of 3C, then the voltage drops slightly
compared to the voltage obtained when the battery is delivering one C. The
curve shows that there is no precise way of determining from voltage alone
the state of the battery. The battery could be 20% discharged or 80% discharged
and it would still deliver almost the same voltage and the same current.
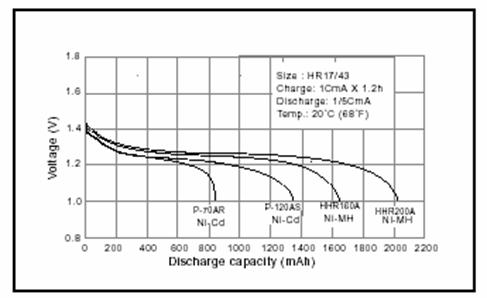
The next figure compares NiCd with NiMH
batteries. The NiCd batteries are rated at 700 and 1200 mAh. The
NiMH batteries at 1600 and 2000 mAh. NiMH can store more energy per
gram than NiCd batteries. Since Cd is toxic, this higher energy density and
greater conceniency has lead to a gradual substitution of NiCd by NiMH batteries.
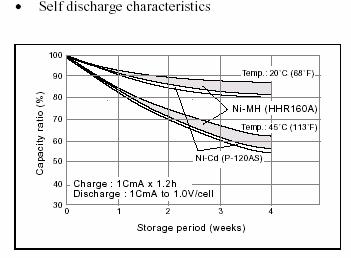
Retention
Charge retention is another important characteristic.
Depending on the battery manufacturer, this parameter can vary greatly. The
curve shows that NiC and NiMH batteries have similar retention ratios, which
vary according to temperature, but are nevertheless similar. At 20 degrees
Celsius the stored charge drops by less than 20% during a month. For other
manufacturers, the retention of NiMH batteries is usually worse than for NiCd
batteries.
Final comparison
The table below compares several different
types of batteries. Gravimetric density shows how many watts hour per Kilogram
can be extracted from a typical battery for each technology. A 100 Watt light
bulb consumes 100 Wh during an hour. While Li-ion
has a better energy density, they are difficult to recharge. NiMH has a better
energy density than NiCd. However, the internal resistance of NiMH batteries
is about 50% higher than for similar NiCd batteries. This means, that when
a current flows the heat dissipated in the battery is higher for NiMH than
for NiCd batteries. The peak load current for NiCd batteries is 20C. For example,
a 600 mAh battery can provide a peak current of 12 Amperes (20 times 0,6 A). The best result is obtained when one C, i.e. 600 mA,
is drained from this battery during continuous use. NiMH batteries can provide
a peak current of 5C. This can be an important parameter in the case of autonomous
robots, if the motors need to drain several amperes when they start accelerating.
If more current is drained, because the robot is stuck and the motors request
more and more energy, for example, the batteries get very warm and can be
damaged.
The table shows also that NiMH batteries
can be recharged, in general, fewer cycles than NiCd batteries.
|
|
NiCd
|
NiMH
|
Lead Acid
|
Li-ion
|
Li-ion polymer
|
Reusable
Alkaline
|
|
Gravimetric Energy Density (Wh/kg)
|
45-80
|
60-120
|
30-50
|
110-160
|
100-130
|
80
(initial)
|
|
Internal Resistance
(includes peripheral circuits) in mW
|
100
to 2001
6V pack
|
200
to 3001
6V pack
|
<1001
12V pack
|
150
to 2501
7.2V pack
|
200
to 3001
7.2V pack
|
200
to 20001
6V pack
|
|
Cycle Life (to 80% of initial capacity)
|
15002
|
300
to 5002,3
|
200
to
3002
|
500
to 10003
|
300
to
500
|
503
(to 50%)
|
|
Fast Charge Time
|
1h
typical
|
2-4h
|
8-16h
|
2-4h
|
2-4h
|
2-3h
|
|
Overcharge Tolerance
|
moderate
|
low
|
high
|
very
low
|
low
|
moderate
|
|
Cell Voltage (nominal)
|
1.25V6
|
1.25V6
|
2V
|
3.6V
|
3.6V
|
1.5V
|
|
Load Current
-
peak
- best result
|
20C
1C
|
5C
0.5C or lower
|
5C7
0.2C
|
>2C
1C or lower
|
>2C
1C or lower
|
0.5C
0.2C or lower
|
|
Operating Temperature (discharge only)
|
-40
to
60°C
|
-20
to
60°C
|
-20
to
60°C
|
-20
to
60°C
|
0
to
60°C
|
0
to
65°C
|
|
Commercial use since
|
1950
|
1990
|
1970
|
1991
|
1999
|
1992
|
Figure 1: Characteristics of commonly used
rechargeable batteries
6. The Memory Effect
Some kind of rechargeable batteries exhibit
the so-called memory effect: i.e. if they are not fully discharged during
several cycles, then the amount of power they can deliver diminishes with
time. It is as if the battery would “remember” that, for example, only 50%
of its capacity has been used many consecutive times and then, when more power
is required, it would not deliver more than 50% of the maximum capacity. NiCd
batteries can exhibit this effect, NiMH batteries also, but to a much smaller
degree.
The memory effect is a chemical transformation
of the electrodes. If a battery is not fully discharged several times, then
large crystals tend to build up at the electrodes. Large crystals diminish
the effectiveness of the chemical reactions in the battery cells. The picture
shows an electron photograph of the changes in the electrodes. To the left
we can see the corny structure of the electrode in its original state. The
porous structure maximizes the surface of the electrode and its contact with
the electrolyte. The chemical reactions take place at the normal rate. In
the middle we see an electrode in which a large crystal has built up. The
effective surface of the electrode has diminished by a large factor and the
chemical reactions are impaired. To the right we can see the same electrodes
after recovery, which is usually done by subjecting the battery to several
cycles of full charge and discharge.


 Electron photograph of a NiCd electrode. To the left, original state of the electrode with
many cadmium hydroxide crystals. In the center a large crystal has
developed and the capacity of the battery has fallen by around 20%. To the right, the state of the reconditioned battery.
Electron photograph of a NiCd electrode. To the left, original state of the electrode with
many cadmium hydroxide crystals. In the center a large crystal has
developed and the capacity of the battery has fallen by around 20%. To the right, the state of the reconditioned battery.
The memory effect arises therefore, when
not all the active material in the battery is recycled by going through a
full charge-discharge cycle regularly. Battery analyzers can help in discharging and charging batteries during several
cycles at low currents. The battery need to be fully discharged several times
automatically. To avoid the memory effect, it is recommended to fully discharge
NiCd batteries at least once a month, and NiMH batteries
at least once every three months.
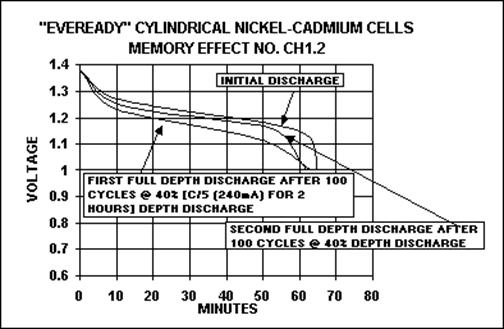
The picture below shows the development
of the memory effect for a NiCd battery. The first discharge takes around
an hour at the full battery rate (C) before voltage drops below 1.1 V. After
100 cycles of partial discharge, a full discharge leads to a noticeable voltage
drop after only 50 minutes. The battery yields now less power as originally.
The next full discharge shows that the battery has recovered a little, although
it is still not as good as originally.
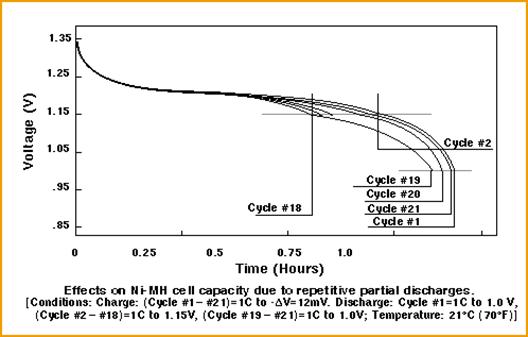
The next picture shows a small memory effect
for NiMH batteries. After 18 cycles of partial discharge (0.75 hours at 1C),
the discharge curve has moved down slightly. Several full discharge cycles
*19 to 21) bring the curve back near to the original discharge curve (cycle
1).
7. Conclusions
A battery is just a device which transforms
chemical energy in an electric current. There are several ways to do this,
according to the type of materials used. In general, a redox (reducing-oxidizing)
pair of reactions is needed. The negative electrode, the anode, oxidizes and
sets electrons free, whereas the cathode reduces and absorbs electrons. The
electrons travel from one electrode to another through a motor or a chip or
whatever device is connected to the power source.
NiCd batteries have been traditionally used
for small robots, since they have been in commercial use since the 1950s.
Their advantages are:
-
fast and simple to recharge
-
can service high peak loads
-
survive many recharge cycles
-
economical, rugged and with long shelf-life
Their disadvantages are their lower energy
density compared to NiM batteries and their toxicity
for the environment.
NiMH batteries are replacing NiCd batteries
due to their higher energy density, but have a shorter life (in terms of charge-discharge
cycles). They cannot deliver as much energy as NiCd for peak loads, and this
can be a disadvantage if a robot occasionally drains much concentrated energy.
They are less prone to suffer the memory-effect than NiCd batteries.